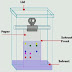
Chromatography is a
modern separation procedure that consists of different physical techniques and
used in many industrial sector especially in the chemical and pharmaceutical sector.
This process is mainly applied for the separation of organic compounds mixture.
Though, a number of other separation processes are there, applied in the
purification process but when it comes to separating two similar physical and
chemical nature organic compounds, this process is the best solution. Those
mixtures cannot be purified through any other methods; only chromatography
process can be applied for the purification.
Chromatography is a
modern separation procedure that consists of different physical techniques and
used in many industrial sector especially in the chemical and pharmaceutical sector.
This process is mainly applied for the separation of organic compounds mixture.
Though, a number of other separation processes are there, applied in the
purification process but when it comes to separating two similar physical and
chemical nature organic compounds, this process is the best solution. Those
mixtures cannot be purified through any other methods; only chromatography
process can be applied for the purification.
In this process the
components of the mixture are segregated in two different phases; on is called
the stationary phase and the other one is called mobile phase. In which
chromatography process the stationary phase is a solid adsorbent and acts like
a column and the mobile phase passes through that column, that process is
called column chromatography. The process of purification takes place depending
on the polarity differences between the components. The technique is components
with more polarity will be adsorbed by the stationary phase and can be
collected separately.
The process of chromatography can be different depending on the nature of the components.
Different kinds of chromatography processes are silica gel chromatography,
aluminum oxide chromatography, flash chromatography, thin layer chromatography
etc. Depending on the nature of the mixture to be purified, the specific
chromatography process is applied. And the stationary phase changes
accordingly. The role of the adsorbents is undeniable in the entire process.
And one of the popular adsorbents for this process is silica gel. Different
silica gel powder mesh like silica gel 60-120 mesh, silica gel 200-230
mesh, and silica gel 230-400 mesh can be used as adsorbents in this process.
Aluminum oxide in the form of powder is also a very good adsorbent used in this
process.
Advantages of
column chromatography:
1. This process is capable of separating
complex mixture of similar physical and chemical nature compounds.
2.
The separation process is much more
cost effective.
3.
It can separate compounds according
to the size and chemical properties.
4.
The process of purification can be
accomplished through a variety of methods.
5.
The recovered products are very pure
in quality.
Getting hold of excellent quality adsorbents could have been
a tough job but with the help of internet it has become extremely easier and
convenient at the same time. There are a number of online adsorbents providers
that offer high quality adsorbents to achieve better outcomes of the separation
process. Since the quality of the adsorbents has a large role to play in this
purification process, purchasing high quality adsorbents from online can prove
to be extremely effective.